Product Quality
Quality assurance / production system that can be done because it is a compact organization
Quality assurance is not limited to quality, but we cooperate with each department to comply with customer requirements and ensure stable manufacturing without difficulty. We carry out activities across departments, from process design development to initial flow management.

Inspection environment
In the inspection process, we pursue the inspection environment desired by our customers and prepare clean rooms and darkroom inspection environments at each base. In addition, if there is a mode that can be confirmed under special conditions, we are constructing the inspection process from the customer's perspective, such as preparing an inspection environment using the actual machine. The clean room is class 10,000 or less (actually 1,000 or less), and we carry out environmental measurement / 5S activities to maintain the environment. Cleanliness ability value. (Class 10000 = JIS standard clean room class standard class 7)
Yamagata factory measured value 300 or less (0.5 μm fine PTCL in 1 ft3))
Sendai factory measured value 200 or less (0.5 μm fine PTCL in 1 ft3)
Shizuoka factory measured value 100 or less (0.5 μm PTCL in 1 ft3)
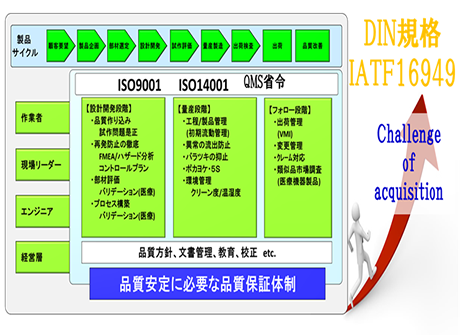
Management System
We are working on a quality management system to deliver products / services to our customers and keep them satisfied. In addition to acquiring the international standard ISO9001 / ISO14001 certification, we pursued the quality required by our customers, such as building a quality management system that requires a QMS ministry ordinance in the field of medical equipment and a high-level perspective. We are working to achieve this while evolving daily.

Information Security Management
Although we have not obtained certification, we will also work on building an information security management system in-house, aiming for a system that gives customers peace of mind. With more than 30 years of experience in delivering to in-vehicle devices and manufacturing medical devices, we have built a quality assurance system from a high-level perspective and are working every day to further improve our level.
Design Review
The design review will be held by the quality assurance department, not the technical department. By holding quality assurance, we pursue design and development from the customer's perspective and while drawing out the development capabilities of the technical department in pursuit of ease of product manufacturing. Customers' needs are maximized by the activities of the sales department. We carry out activities across departments from process design development to initial flow management.


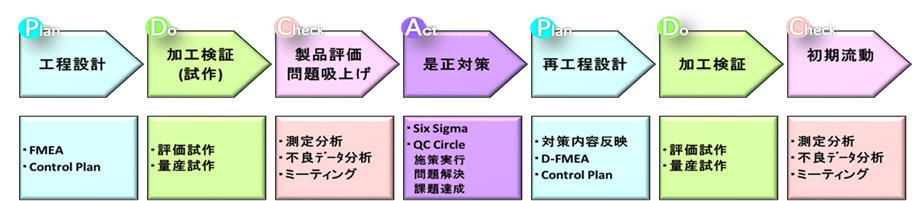
Initial Flow Management
When constructing a new process, various unexpected things may occur, so we manage by properly using the tools to be used according to the target product type and management items. At our company, each department jointly implements preventive measures activities using FMEA and risk analysis. After that, the dimensional control of the designated place, the occurrence of defective products, and the period until the target value of productivity can be achieved are set as the monitoring and measurement period, and if there is a failure or problem, each department will cooperate and make improvements. .. If there are other customer requests, we will carry out activities in accordance with the requirements so as to achieve the requirements.
Quality verification equipment
Quick vision or 3D measuring machines are installed at each site, and it is possible to verify the dimensions of processed products at each site. Regarding the color verification of screen printed products, we guarantee by using a color luminance meter and managing the measured values.

Product Quality
Quality assurance / production system that can be done because it is a compact organization
Quality assurance is not limited to quality, but we cooperate with each department to comply with customer requirements and ensure stable manufacturing without difficulty. We carry out activities across departments, from process design development to initial flow management.


Inspection environment
In the inspection process, we pursue the inspection environment desired by our customers and prepare clean rooms and darkroom inspection environments at each base. In addition, if there is a mode that can be confirmed under special conditions, we are constructing the inspection process from the customer's perspective, such as preparing an inspection environment using the actual machine. The clean room is class 10,000 or less (actually 1,000 or less), and we carry out environmental measurement / 5S activities to maintain the environment. Cleanliness ability value. (Class 10000 = JIS standard clean room class standard class 7)
Yamagata factory measured value 300 or less (0.5 μm fine PTCL in 1 ft3))
Sendai factory measured value 200 or less (0.5 μm fine PTCL in 1 ft3)
Shizuoka factory measured value 100 or less (0.5 μm PTCL in 1 ft3)

Management System
We are working on a quality management system to deliver products / services to our customers and keep them satisfied. In addition to acquiring the international standard ISO9001 / ISO14001 certification, we pursued the quality required by our customers, such as building a quality management system that requires a QMS ministry ordinance in the field of medical equipment and a high-level perspective. We are working to achieve this while evolving daily.

Information Security Management
Although we have not obtained certification, we will also work on building an information security management system in-house, aiming for a system that gives customers peace of mind. With more than 30 years of experience in delivering to in-vehicle devices and manufacturing medical devices, we have built a quality assurance system from a high-level perspective and are working every day to further improve our level.

Design Review
The design review will be held by the quality assurance department, not the technical department. By holding quality assurance, we pursue design and development from the customer's perspective and while drawing out the development capabilities of the technical department in pursuit of ease of product manufacturing. Customers' needs are maximized by the activities of the sales department. We carry out activities across departments from process design development to initial flow management.

Initial Flow Management
When constructing a new process, various unexpected things may occur, so we manage by properly using the tools to be used according to the target product type and management items. At our company, each department jointly implements preventive measures activities using FMEA and risk analysis. After that, the dimensional control of the designated place, the occurrence of defective products, and the period until the target value of productivity can be achieved are set as the monitoring and measurement period, and if there is a failure or problem, each department will cooperate and make improvements. .. If there are other customer requests, we will carry out activities in accordance with the requirements so as to achieve the requirements.

Quality verification equipment
Quick vision or 3D measuring machines are installed at each site, and it is possible to verify the dimensions of processed products at each site. Regarding the color verification of screen printed products, we guarantee by using a color luminance meter and managing the measured va